Alliance for Cancer Gene Therapy (ACGT) has awarded a research grant to Crystal Mackall, MD (Stanford University), to develop a sophisticated cell and gene therapy that can overcome some of the roadblocks presented by solid tumors.
This grant will support Dr. Mackall’s advancement and testing of a CAR T-cell therapy that uses a unique remote-control feature for improved safety and effectiveness.
CAR T-cell therapy is a type of cell and gene therapy that modifies a patient’s T cells to more effectively find cancer cells expressing a specific protein. In 2017, the U.S. Food and Drug Administration (FDA) approved the first CAR T-cell therapy for cancer. That approval of Kymriah for children and young adults with acute lymphoblastic leukemia was the first of six approved CAR T-cell therapies for various types of blood cancer.
While progress is being made, there has not yet been a breakthrough in developing CAR T-cell therapies for solid tumors such as lung cancer, pancreatic cancer and ovarian cancer. Solid tumors are much harder to treat than blood cancers and require more complex strategies.
The challenges of CAR T-cell therapy for solid tumors.
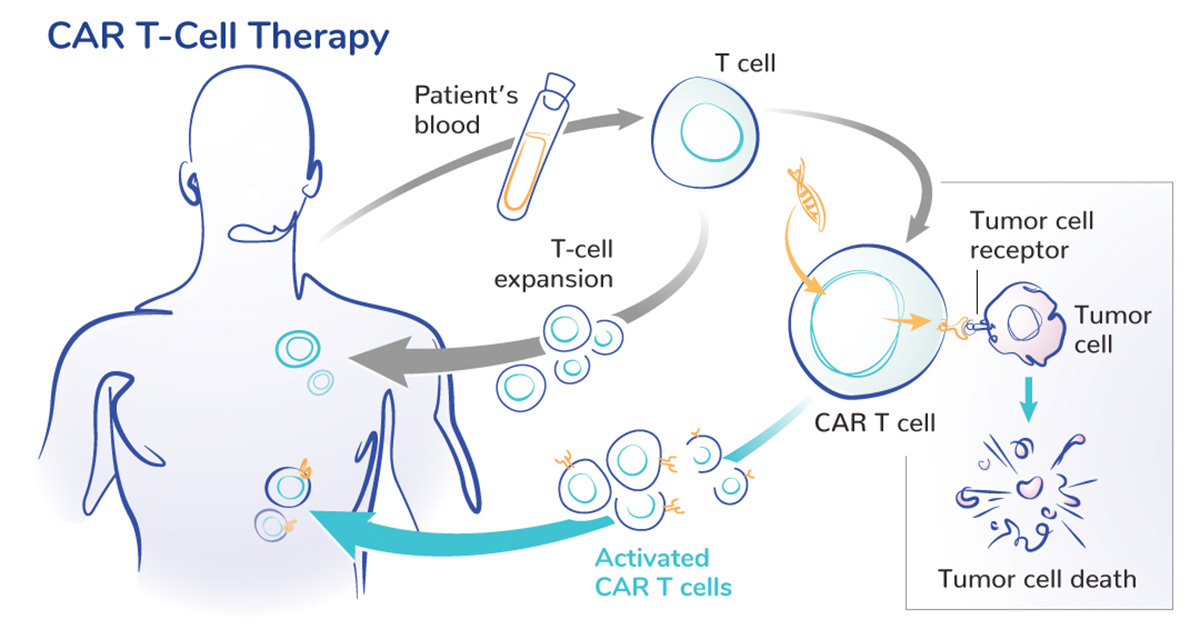
There are several reasons why this science has not achieved the same success.
First, finding a protein target for solid tumors is difficult. Most of the possible targets are also expressed by healthy tissue, albeit at lower levels.
“We worry about going after these targets with strong T cells because it could cause harm to healthy tissue and cause toxicities for patients,” Dr. Mackall said.
Second, solid tumors often have an immunosuppressive microenvironment that can exhaust CAR T cells before they kill enough cancer cells to stop the tumor.
“If we identify a strong protein target and engineer a potent CAR T cell, we may have toxicities,” Dr. Mackall says. “That’s where I think the field has stalled a bit.”
How does Dr. Mackall’s remote-control CAR T-cell therapy work?
Dr. Mackall’s remote-control therapy – called “SNIP-CAR” – has several unique features.
First, the engineered cells begin in an “off” state, and the patient receives a medication to turn them on. If the patient begins to experience toxicities, scientists can pause the medication to turn the CAR T cells off. As the patient recovers, the medication can be readministered to turn their engineered cells back on.
Scientists can also change the dose or frequency of the medication to “tune” the CAR T cells’ activity. Ideally, the cells will only attack cancer cells expressing the protein target while ignoring healthy tissue expressing the protein at a lower level.
“When we had a mouse model where the big problem was shared expression of the protein target between tumor cells and healthy tissue cells, by tuning the dose down, it tuned the amount of antigen needed to activate the CAR T cells. We saw an anti-tumor effect but not toxicity. That’s the other part of these remote-control cells. We may be able to distinguish better between normal tissue and tumor cells.” — ACGT Research Fellow Crystal Mackall, MD (Stanford University)
Giving CAR T cells ‘rest’ time.
There was one unexpected result from early testing of the SNIP-CAR therapy: The “off” state was also a “rest” state, giving the cells time to recover their energy. This rest period made the engineered cells more effective when turned back on.
“So not only are you diminishing toxicity, but you’re also allowing the cells to potentially recover,” Dr. Mackall said. “The cells are more potent for the long-haul. They don’t exhaust themselves. They’re good long-term, which is what you need against solid tumors.”
ACGT’s support of Dr. Mackall’s cell and gene therapy research.
Funding from ACGT will support a phase 1 clinical trial testing the SNIP-CAR therapy in humans, which is designed to target the protein B7H3. This protein is expressed by most solid tumors.
“That’s the good news,” Dr. Mackall said of how common B7H3 is in solid tumors. “The bad news is it’s also expressed on some normal tissue. So, this is a good test for this project since standard B7H3-targeting CAR T cells would cause toxicities, and one goal of the remote-control therapy is controlling toxicity.
“Another goal is that the cells are more potent than standard B7H3-targeting CAR T cells. How do we measure that? One way is how long they last. Most of these CAR T cells last a few weeks and then disappear. If we see long-term persistence of these cells for several months, that’d be a real win.”
This is the second ACGT research grant awarded to Dr. Mackall, who received her first in 2016. That grant supported Dr. Mackall’s research and development of a CAR T-cell therapy for people with osteosarcoma (bone cancer) and neuroblastoma (nerve cell cancer). This research led to a clinical trial for these two types of cancer and translated into a clinical trial for children with a rare and aggressive brain tumor called diffuse midline glioma.
“ACGT has a deep understanding of the science,” Dr. Mackall said. “When reviewing potential projects to fund, they know which ones have the most potential and promise. There is deep expertise and fearlessness in taking risks. They don’t want to fund the same old projects.”



