Welcome to our July 2024 newsletter.
|
Cancer survivor story: Lauri Adami’s road to CAR T-cell therapy.
|
Laurie Adami’s 12-year battle with follicular lymphoma included six lines of treatment that did not successfully put her in a long-term remission. She tried FDA-approved therapies and enrolled in clinical trials, yet none worked – until CAR T-cell therapy.
On July 16, 2018, Laurie received her CAR T cells. One month later, her scans showed no signs of the follicular lymphoma that had upended her life for more than a decade.
She has been cancer-free ever since.
Today, Laurie celebrates the sixth anniversary of receiving her life-saving CAR T-cell therapy. Read the full story of Laurie Adami’s fight with cancer and journey to cell and gene therapy.
|
ACGT funding development of remote-control CAR T cells.
|
Alliance for Cancer Gene Therapy (ACGT) has awarded a $500,000 grant to Crystal Mackall, MD (Stanford University), to support the development of a CAR T-cell therapy with a unique remote-control feature. This attribute for the engineered cells could help them overcome some of the challenges caused by solid tumors, such as patient toxicities and T-cell exhaustion.
Dr. Mackall’s CAR T cells have an “on/off switch” that allows scientists to temporarily turn off the engineered cells when patients experience toxicities. Once toxicities subside, the cells can be turned back on to continue fighting the patient’s cancer.
Solid tumors often have an immunosuppressive environment that exhausts T cells. The “off period” for Dr. Mackall’s remote-controlled CAR T cells can provide “rest” time to recover their energy.
This is the second ACGT research grant awarded to Dr. Mackall, who received funding from the foundation in 2016 to develop a CAR T-cell therapy for pediatric osteosarcoma and neuroblastoma. Read more about her new ACGT research grant on our website.
|
|
|
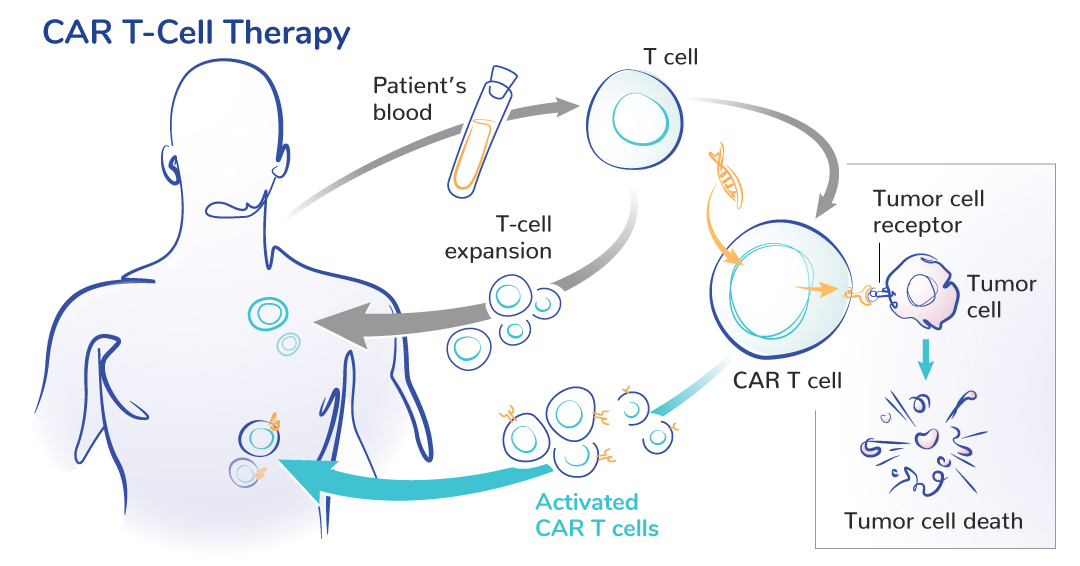
CAR T cells are immune cells engineered to find and attack cancer. They are created from a patient’s own T cells, which are genetically modified to search for cancer cells expressing a specific protein.
Once the modified cells are returned to the patient’s blood, they multiply to create more CAR T cells capable of fighting cancer.
There are six FDA-approved CAR T-cell therapies for blood cancers. A focus now is on developing CAR T cells for solid tumors, as explained in this video by ACGT Research Fellow Carl June, MD (University of Pennsylvania).
|
|
|
Swim Across America swimmers hit the water to raise money for cell and gene therapy research.
|
|
|
The 18th annual Swim Across America-Fairfield County (SAA-FC) open water swim was held June 29, on the Long Island Sound in Stamford, Connecticut. The event benefits ACGT’s mission to fund cancer cell and gene therapy research.
Since the inaugural open water swim in 2007, SAA-FC has raised nearly $6 million to benefit ACGT. Donate today on the SAA-FC website.
Picture highlights from the 2024 event are on our website. All pictures are courtesy of Swim Across America-Fairfield County.
|
|
|
Your legacy, our future: How today’s gifts shape tomorrow’s cures.
|
Imagine a world where the breakthroughs of today pave the way for cancer cures that will save lives tomorrow.
When you leave a legacy gift to ACGT, you are not just making a donation – you are making a difference for future generations. Your gift will ensure the scientific discoveries of today lead to the life-saving treatments and cures of tomorrow, transforming the landscape of cancer care for years to come.
Including ACGT in your estate plans means you are empowering the next wave of scientific research that will bring hope and healing to countless families.
Will you help create a cancer-free future? Contact Keri Eisenberg, CFRE, ACGT Chief Philanthropy Officer, at keisenberg@acgtfoundation.org to explore how you can leave a lasting legacy that changes the world.
|
|
|
Sarcoma Awareness Month: Q&A with a sarcoma expert.
|
July is Sarcoma Cancer Awareness Month. Sarcomas are a group of cancers that form in bones and nerve cells. Each year, 13,000-16,000 people in the U.S. are diagnosed with sarcomas, and approximately 5,000 people die annually from these cancers.
Wendy Walk, a partner of ACGT, is a nonprofit organization dedicated to raising money for sarcoma research. Alexandra, Matt and Jackie Landes formed Wendy Walk to honor the courageous sarcoma cancer battle of their late mother, Wendy Landes. There have been more than 40 walks and more than 10,000 walkers since the inaugural event in 2010, and Wendy Walk has raised $610,000 to benefit ACGT.
ACGT Research Fellow Stephen Gottschalk, MD (St. Jude Children’s Research Hospital), received grant funding to develop a dual-targeted CAR T-cell therapy for pediatric sarcomas. His grant from ACGT was funded in part by Wendy Walk.
Dr. Gottschalk spoke with ACGT for #SarcomaAwarenessMonth about the challenges of developing novel therapies for sarcomas. Below is an excerpt, and the full interview is available on our website.
What are some of the challenges with advancing cell and gene therapies for sarcomas?
Dr. Gottschalk: “The biggest hurdle is the limited number of biopsy specimens and, in general, the lack of patient samples to analyze and research. For rarer cancers like sarcomas, there are just fewer patients to study whereas if you are studying the biology of lung cancer, there are hundreds of patients.
Secondly, in a world of limited resources, more research funds are allocated to the most common cancers rather than rare cancers like sarcomas. So, we need more dollars directed toward sarcoma research.”
ACGT has awarded three research grants focusing on sarcomas, and the foundation continues to fund research for less-common cancers. Help us continue to fund cell and gene therapy research for rare cancers like sarcomas.
|
|
|
ACGT scientists honored with Harvard Medical School award.
|
ACGT Research Fellows and Scientific Advisory Council members Carl June, MD (University of Pennsylvania), and Michel Sadelain, MD, PhD (Memorial Sloan Kettering Cancer Center), have received the 2024 Warren Alpert Foundation Prize for making transformational research discoveries leading to the creation of groundbreaking CAR T-cell therapies for cancer.
Drs. June and Sadelain used grant funding from ACGT to conduct the research that led to this remarkable achievement, which Dr. June talks about in this video.
The Warren Alpert Foundation Prize is administered by Harvard Medical School, which will host a scientific symposium on Oct. 10 when the award winners will be recognized. Including the 2024 prize, the foundation has awarded more than $8 million to 83 individuals.
|
|
|
The latest on cancer cell and gene therapy from around the world.
|
|
|
|