The term “immunotherapy” has quite a wide scope in cancer treatment. A lot of different therapies fall within the umbrella of immunotherapy, and two of the most successful are CAR T-cell therapies and immune checkpoint inhibitors.
What differentiates these two types of immunotherapy? Are they similar in how they help the immune system? Where do these two therapies stand in terms of effectiveness and approval for solid tumors?
And finally, can these two types of immunotherapy work together to treat cancer?
This page on the Alliance for Cancer Gene website compares CAR T-cell therapies and immune checkpoint inhibitors. If this is your first time visiting Alliance for Cancer Gene Therapy – or even if you’re a returning visitor – we encourage you to sign up to our monthly email newsletter and other communications offerings. This will keep you up-to-date on news and events in the world of cancer cell and gene therapy.
What is CAR T-cell therapy?
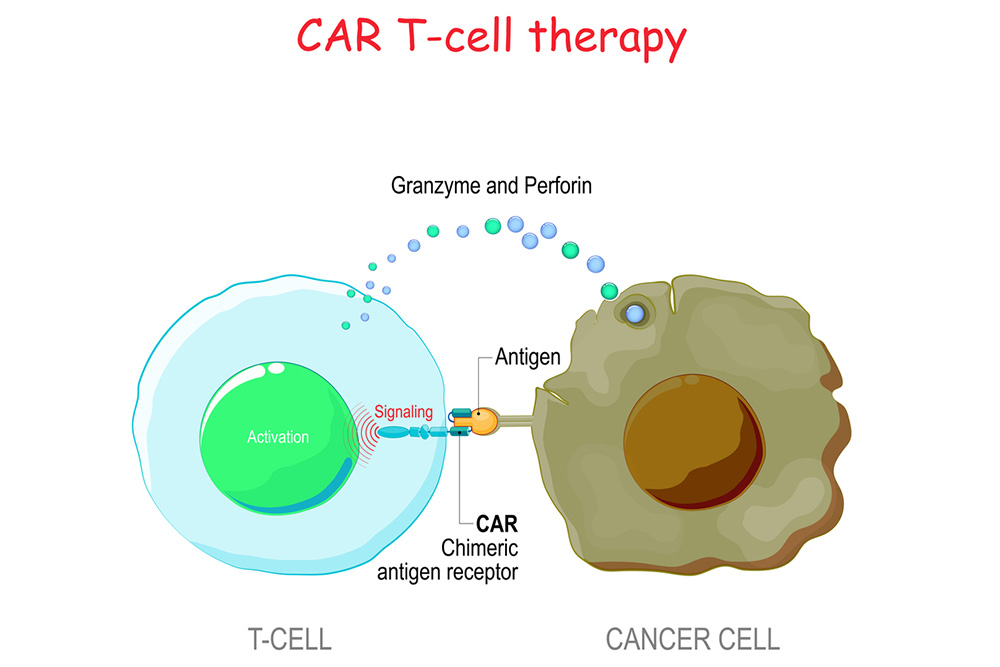
CAR T-cell therapy is a type of cell and gene therapy that elevates the immune system cells against cancer. T cells and natural killer (NK) cells are the body’s primary defenders against viruses, infections and other harmful elements. However, they often stumble in locating and killing cancer cells.
CAR stands for chimeric antigen receptor, which is a special laboratory-created receptor designed to bind to target proteins on cancer cells. Doctors can remove patients’ T cells and NK cells to have a receptor added. Scientists develop the receptors to link with proteins highly expressed on cells of a specific cancer.
CAR T cells and CAR NK cells essentially are given tunnel vision to look for cancer cells. Once infused back into the bloodstream, the upgraded cells replicate to grow an army of immune system cells to better fight cancer.
Approved CAR T-cell therapies
There are currently six FDA-approved CAR T-cell therapies for cancer. They are all designed for specific blood cancers and primarily approved for use after multiple failed attempts with other therapies to control the cancer, like chemotherapy or radiation.
The six approved CAR T-cell therapies are:
- Kymriah® for non-Hodgkin lymphoma (and pediatric acute lymphoblastic leukemia)
- Yescarta® for non-Hodgkin lymphoma, which includes large B-cell lymphoma
- Tecartus® for mantle cell lymphoma and acute lymphoblastic leukemia
- Breyanzi® for types of non-Hodgkin lymphoma, including large B-cell lymphoma
- Abecma® for multiple myeloma
- Carvykti® for multiple myeloma
Two of these CAR T-cell therapies have also been approved for earlier use: Kymriah® for pediatric acute lymphoblastic leukemia; and Yescarta® as a second-line treatment for relapsed or refractory large B-cell lymphoma (LBCL).
Side effects of CAR T-cell therapy
The critical side effect from CAR T-cell therapy is cytokine release syndrome (CRS). When infused CAR T cells enter the body, they release cytokines, or cell messengers directing the body’s immune system. The release of these cytokines can cause inflammation and disrupt normal body functioning. Doctors and patients manage CRS as part of the CAR T-cell process. Importantly, CRS can be an indicator that CAR T therapy is working.
Another possible side effect is immune effector cell-associated neurotoxicity syndrome (ICANS). This syndrome changes the patient’s nervous system.
Additional side effects from CAR T-cell therapy include:
- Chills
- Dizziness
- Fatigue
- Fever
- Headaches
- Nausea
What is an immune checkpoint inhibitor?
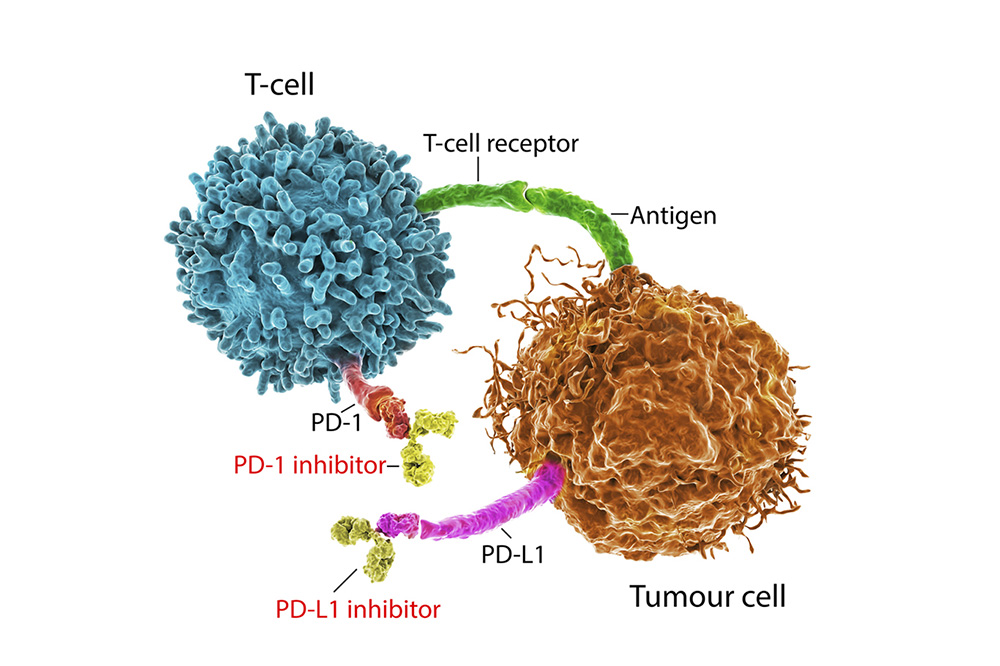
Immune checkpoint inhibitors are immunotherapy drugs that block cancerous protein actions on either cancer cells or fighter T cells. Cancer cells have masking protein receptors that can trick T cells into leaving them alone. Immune checkpoint inhibitors neutralize these protein receptors.
An example of the target protein receptor is programmed death-ligand 1 (PD-L1). This receptor links with the T cells’ programmed cell death protein 1 (PD-1). The role of PD-1 is to look for infections, viruses or cells harming the body, such as cancer. The issue is PD-L1 subdues PD-1 when T cells interact with some cancer cells.
Immune checkpoint inhibitors serve as a wall between the protein receptors. They slice through the connection, which allows T cells to recognize cancer cells as harmless.
Examples of immune checkpoint inhibitors
The U.S. Food and Drug Administration (FDA) has approved many immune checkpoint inhibitors for various cancers. A few of the accepted options are:
- Opdivo® (brand name for nivolumab) – a PD-1/PD-L1 checkpoint inhibitor approved for bladder cancer, colorectal cancer, esophageal cancer, gastric cancer, head and neck cancer, kidney cancer, liver cancer, lung cancer, lymphoma, melanoma and mesothelioma
- Yervoy® (brand name for ipilimumab) – a CTLA-4/B7 checkpoint inhibitor approved for liver cancer, lung cancer, melanoma and mesothelioma
- Keytruda® (brand name for pembrolizumab) – a PD-1/PD-L1 checkpoint inhibitor approved for bladder cancer, breast cancer, cervical cancer, colorectal cancer, squamous cell carcinoma and Merkel cell carcinoma, esophageal cancer, head and neck cancer, kidney cancer, liver cancer, lung cancer, lymphoma, melanoma and stomach cancer
- Imfinzi® (brand name for durvalumab) – a PD-1/PD-L1 checkpoint inhibitor approved for bladder cancer and lung cancer
- Tecentriq® (atezolizumab) – a PD-1/PD-L1 checkpoint inhibitor approved for bladder cancer, breast cancer, liver cancer, lung cancer and melanoma
Side effects of immune checkpoint inhibitors
Immune checkpoint inhibitors have a few of the same side effects as CAR T-cell therapy, such as fatigue, headaches, and fever. The body adjusts to the addition of therapeutic drugs that alters the immune system’s process, which causes an internal reaction and requires an adjustment period.
Additional side effects for immune checkpoint inhibitors are:
- Skin irritation
- Issues with the gastrointestinal tract, such as swallowing problems and diarrhea
- Joint swelling and muscle cramping
- Pneumonitis (inflammation of the lungs)
Can immune checkpoint inhibitors and CAR T-cell therapies work together?
The concept of pairing immune checkpoint inhibitors and CAR T cells together is still in testing and development. However, there appears to be some merit to the idea of using two forms of immunotherapy to make cancer cells vulnerable to the immune system.
Providing the immune system with CAR enhancements and adding immune checkpoint blockade drugs means these powerful T cells won’t be fooled by the cancer cells’ misleading protein receptors.
Some of the latest research even affirms there can be collaboration between the two therapies. As reported in the medical journal American Association for Cancer Research, the protein tyrosine phosphatase PTP1B is a protein receptor on the surface of T cells, and it is linked to T cell suppression against tumors.
Researchers tested an anti-PTP1B drug with a CAR T-cell therapy for breast cancer. The combination amplified the ability of CAR T cells to infiltrate the tumor in studies on mice models.
Page sources
- Immunomodulators: Checkpoint Inhibitors, Cytokines, Agonists, and Adjuvants. Cancer Research Institute. Retrieved from: https://www.cancerresearch.org/en-us/immunotherapy/treatment-types/immunomodulators-checkpoint-inhibitors. Accessed: 03/18/2022.
- Side Effects of Immunotherapy. American Society of Clinical Oncology. Retrieved from: https://www.cancer.net/navigating-cancer-care/how-cancer-treated/immunotherapy-and-vaccines/side-effects-immunotherapy. Accessed: 03/22/2022.
- PTP1B Is an Intracellular Checkpoint that Limits T-cell and CAR T-cell Antitumor Immunity. American Association for Cancer Research. Retrieved from: https://aacrjournals.org/cancerdiscovery/article/12/3/752/681920/PTP1B-Is-an-Intracellular-Checkpoint-that-Limits-T. Accessed: 03/22/2022.



