
Alliance for Cancer Gene Therapy is committed to supporting and advancing the next generation of cancer treatment. This evolved approach involves using the power of gene therapy to either strengthen the immune system or weaken cancer cells.
How does gene therapy work, though?
What is the process of cancer gene therapy? What are the steps to gene therapy? What exactly is gene therapy?
Our breakdown of this treatment should help you better understand the science behind cancer gene therapy, including the process patients go through to receive this promising therapy.
Once you’ve finished reading, please consider making a financial gift to Alliance for Cancer Gene Therapy to help continue our quest for a cure. Our mission is to fund cell and gene therapy research projects that help the immune system attack cancer or target cancer cells directly. Your support will help advance the most innovative and impactful translational research in gene therapies.
As more people understand gene therapy and how it helps treat cancer, there will be more recognition of the need for funding to support critical scientific research.
What is gene therapy?
Gene therapy is an element of cancer cell and gene therapy in which researchers alter the DNA and genetic function of immune cells or cancer cells. Researchers are also examining the tumor microenvironment – the environment cancer builds around it to support its growth and block the immune system – of different types of cancers to learn how they form and what differentiates cancer cells from healthy tissue.
These cellular differences usually manifest in altered, added, or deleted genes to create an environment supporting cancer growth. Gene therapy is a technique to target these changes and treat diseases through engineered therapeutic agents, such as CAR T cells.
What is a gene?
Genes are small sections of DNA, which contain instructions for proteins that control biological function. The body needs up to 400,000 proteins to function properly.
Genes can be altered or mutated, which changes the production and expression of proteins. Mutated genes can throw off the body’s sensitive balance, leading to issues of overproduction and cell replication.
This genetic alteration or mutation is the first step in the development of cancer. However, each cancer expresses these genetic alterations in its own unique ways – usually with different genes affected resulting in the suppression or overexpression of different proteins. Researchers are learning which genetic changes are associated with each cancer and are developing effective gene-targeted therapies with funding from Alliance for Cancer Gene Therapy.
Types of gene therapy for cancer
Gene therapy aims to control the altered genes or genetic mutations of a cancer to prevent the cancer’s growth. This approach to using our own cells and genes to treat cancer is called somatic gene therapy. This type of gene therapy does not impact germ-line cells in the reproductive system, meaning none of the genetic changes can be passed on to other family members.
There are four types of somatic gene therapy: gene editing; gene replacement; gene addition; and gene inhibition.
Gene editing is correcting the cell’s gene to fix the imbalance – by snipping out the faulty part of the gene and changing the cancer’s DNA. This type of gene therapy may correct the alteration rather than trying to remove it.
Gene replacement is just that: replacing the faulty or nonworking gene with a healthy copy of it. This type of gene therapy is another form of trying to fix the genetic change rather than trying to remove it.
Gene addition is adding novel genetic code to a different cell – usually an immune system fighter cell – to help it combat the protein linked to the damaged gene. CAR T-cell therapy is an example of gene addition. This form of gene therapy isn’t adding a copy of an already-existing gene but rather an entirely new gene – usually with the intent of killing the cancer cell via the immune system. Doctors may also add a new gene directly to the cancer cell that causes the cancer cell to commit apoptosis (kill itself).
Gene inhibition simply shuts down the faulty gene. This can either kill the cell or prevent it from acting in a cancerous manner, such as growing and replicating exponentially.
Steps of gene therapy
Gene therapy is a new and potentially curative approach to treating cancer, but researchers still have so much to learn. While the steps below may seem straightforward, each part of the process requires years of study to develop the technologies.
Researchers must first identify the gene and protein linked to the cancer. The next steps are:
- Create or isolate a normal copy of the gene
- Develop a viral vector to carry genetic material into cells
- Insert the gene-carrying vector into the body
- The viral vector delivers the gene to the tumor microenvironment or immune system cells
- The vector inactivates to avoid any further (potentially harmful) activity
- The inserted gene either inactivates, fixes, replaces, or attacks the faulty gene
Steps of CAR T-cell therapy
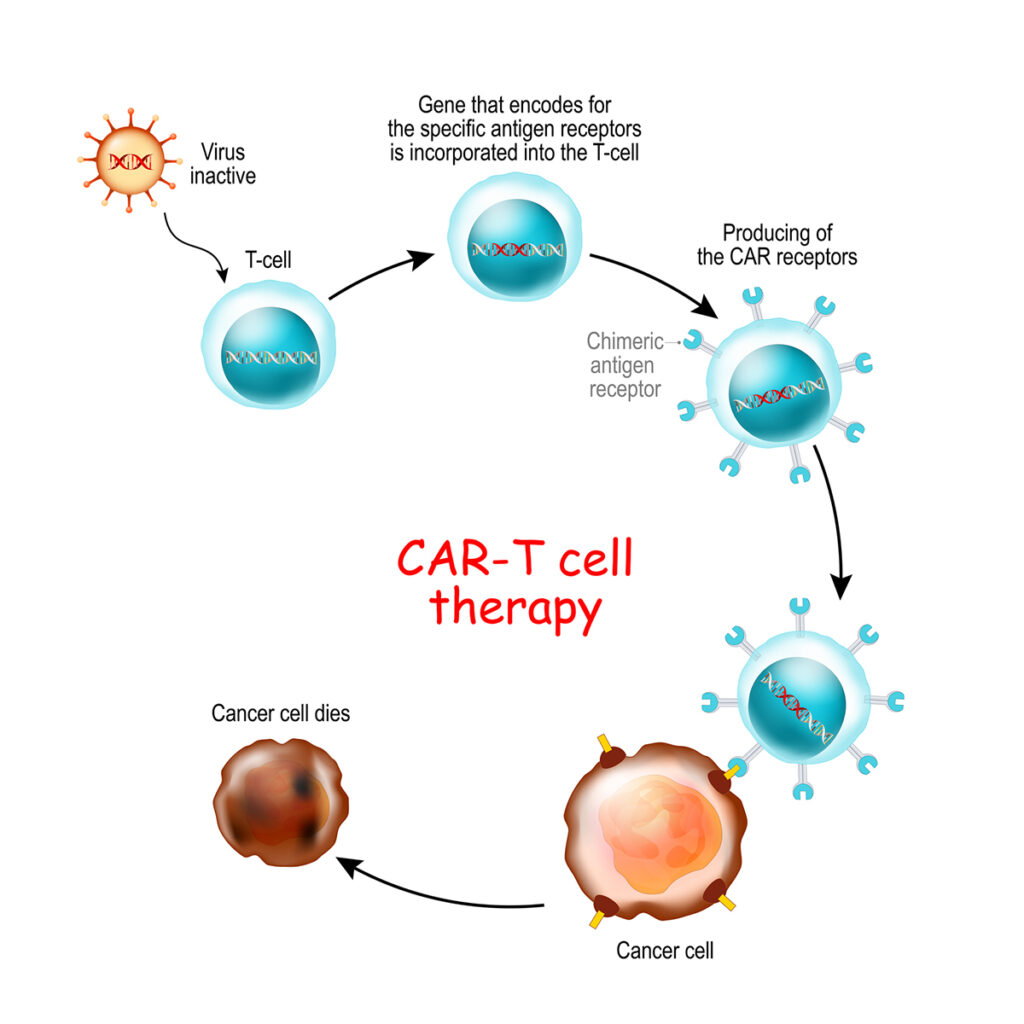
CAR T-cell therapy has a slightly different process than more direct forms of gene therapy. CAR T cells are lab-generated fighter cells with specific, anti-cancer genetic code. Adding this genetic code is the gene therapy component of CAR T-cell therapy. CAR stands for “chimeric antigen receptor,” which is the new genetic code added to the T cells.
There are six CAR T-cell therapy agents approved by the U.S. Food and Drug Administration for different blood cancers. These approvals validate CAR T cells as an effective form of cancer gene therapy to improve patient life expectancy.
Doctors first draw blood from a patient and separate the T cells, which are white blood cells leading the immune system’s defense against viruses, diseases and more unwanted intruders. T cells aim to protect the body from cancer, but they’re often ineffective at doing so.
The process of drawing blood from patients and separating the T cells is called apheresis.
After removing T cells from the body, the steps of CAR T-cell therapy are:
- Identify a protein biomarker (overexpressed protein) on the genetically damaged cancer cells
- Create a RNA strand called a chimeric antigen receptor (CAR) coded to look for the protein biomarker
- Add the chimeric antigen receptors to the extracted T cells
- Multiply the new CAR T cells in the laboratory
- Reinsert the newly motivated CAR T cells into the body through infusion
A similar process occurs for CAR NK-cell therapy. Scientists create chimeric antigen receptors to strengthen natural killer (NK) cells, another white blood cell of the immune system.
How long does CAR T-cell therapy take?
There are six CAR T-cell therapies approved for types of three blood cancers: myeloma, leukemia and lymphoma. CAR T-cell therapy infusions can take place in an inpatient or outpatient care setting, but the patient must be closely monitored at all times.
CAR T-cell therapy can lead to side effects, most notable cytokine release syndrome.
The entire CAR T-cell process lasts approximately one month, not including the recovery time after treatment:
- First, doctors must remove the patient’s T cells, work on them in a lab, and infuse them back into the body. This step usually takes a few weeks to add the CAR to the T cells and multiply them.
- The CAR T cells are frozen and returned to the hospital or cancer center where the patient is treated.
- Infusion of CAR T cells back into the patient takes around one hour, according to the Mayo Clinic’s website.
- After receiving the CAR T-cell infusion, the medical staff monitors the patient for the next few weeks to watch for side effects caused by the enhanced T cells rapidly expanding in the body and killing the cancer cells.
For the first seven days after receiving the CAR T-cell infusion, patients must remain under medical supervision. For weeks 2-4 of the post-infusion timeline, patients must remain within a short drive of their medical facility to respond to any issues.
The total recovery period from CAR T-cell therapy is usually 2-3 months following infusion, according to the Dana-Farber Cancer Institute.
There are several studies for CAR T-cell therapies for cancer. Participating in a clinical trial helps advance cell and gene therapy research and can advance much-needed therapies to more patients in need. Visit our clinical trials page for information or go to the clinicaltrials.gov database.
Page sources
- What Is Gene Therapy? How Does It Work? U.S. Food and Drug Administration. Retrieved from: https://www.fda.gov/consumers/consumer-updates/what-gene-therapy-how-does-it-work. Accessed: 01/25/2022.
- How CAR T-Cell Therapy Works. Dana-Farber Cancer Institute. Retrieved from: https://www.dana-farber.org/cellular-therapies-program/car-t-cell-therapy/how-car-t-cell-therapy-works/. Accessed: 01/25/2022.
- Gene Therapy. Mayo Clinic. Retrieved from: https://www.mayoclinic.org/tests-procedures/gene-therapy/about/pac-20384619. Accessed: 03/07/2022.



