Kymriah, the brand name for the CAR T-cell therapy tisagenlecleucel, is already an FDA-approved cell and gene therapy for multiple types of blood cancer. The therapy has helped many patients with specific types of leukemia and lymphoma tremendously, improving their survival rates and giving them hope for long and healthy futures.
One of the next questions is whether the administration of this therapy can be adjusted to better help a larger number of these cancer patients.
Researchers uncovered that administering higher doses of Kymriah improved the overall survival of young leukemia patients without adding toxic side effects. This news, which was published by the American Society of Hematology, may adjust the administered dose of Kymriah to the highest FDA-approved level for children, teenagers and young adults diagnosed with acute lymphoblastic leukemia (ALL).
Alliance for Cancer Gene Therapy regularly publishes news about the field of cancer cell and gene therapy. If you’d like to stay informed via a monthly email newsletter, please sign up through our contact form page. This email newsletter provides a roundup of the most recent cell and gene therapy-related articles published on the Alliance for Cancer Gene Therapy website and elsewhere in the scientific community.
Kymriah for acute lymphoblastic leukemia
On Aug. 30, 2017, Kymriah became the first CAR T-cell therapy approved for any type of cancer. The U.S. Food and Drug Administration (FDA) approved the treatment for patients up to 25 years old with acute lymphoblastic leukemia.
While there are now six CAR T-cell therapies approved for various blood cancers, Kymriah and Tecartus are the only two therapies approved for a type of leukemia. Tecartus is approved for adult patients (18 years and older) with relapsed or resistant precursor ALL.
The approval of Kymriah was much-needed, as ALL is the most common childhood cancer. Chemotherapy usually suffices in stopping and killing the disease, but around 20% of patients either don’t respond to chemotherapy or relapse soon after the treatment ends.
These patients are the ones who currently benefit from CAR T-cell therapy, which is an alternative to a stem-cell transplant.
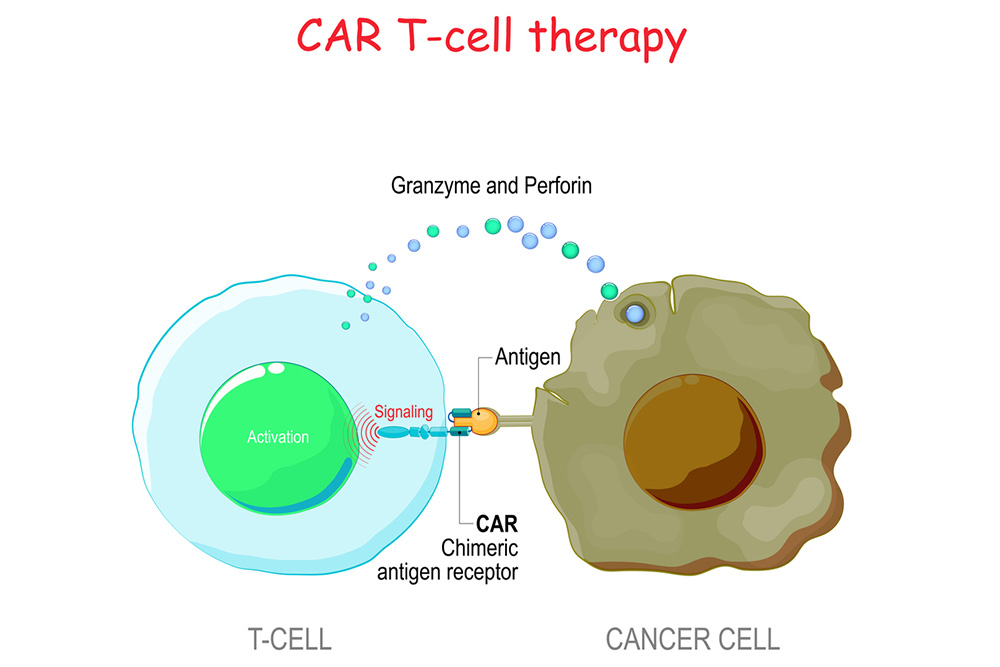
CAR T-cell therapy involves taking out patients’ T cells and modifying them with a laboratory-created protein receptor, called a chimeric antigen receptor (CAR). This protein receptor trains the T cells to search for any cells expressing a specific cancerous protein. This method helps the T cells focus on sniffing out and fighting cancer cells, which often go unnoticed by T cells that don’t have this chimeric antigen receptor.
Increased dosage of Kymriah leads to improved survival
Liora Schultz, MD, a pediatric oncologist at Stanford Children’s Health Lucile Packard Children’s Hospital, was the lead author for examining the impact of a higher dose of Kymriah. She and others investigated whether higher doses of the therapy could improve one-year survival rates among young patients with acute lymphoblastic leukemia (ALL).
“In the past, we did not have data to guide clinical decisions around commercial CAR T-cell dosing and didn’t know if higher doses would affect toxicity and compromise outcomes, or support enhanced anti-leukemia effect. This data has direct clinical applicability, as it supports use of higher dosing, as available, within the approved tisagenlecleucel dose range.” — Liora Schultz, MD, a pediatric oncologist at Stanford Children’s Health Lucile Packard Children’s Hospital
Kymriah is approved at different dosing ranges based on patient weight. One of the approved ranges is 0.2-5.1 million CAR T cells/kg for patients weighing 50 kilograms or less, which equals 110 lbs and applies mostly to young children.
The study involved 185 patients who were 26 years old or younger. Each participant had relapsed or resistant acute lymphoblastic leukemia.
The two dose ranges tested were 0-1.3 million cells/kg and 2.4-5.1 million cells/kg, both within the U.S. Food and Drug Administration’s approved range. Patients receiving the higher dose range had much higher survival rates than patients receiving the lower dose range:
- The higher dose range had an 86% 1-year survival rate
- The lower dose range had a 59% 1-year survival rate
Additionally, there was not any evidence of increased toxicity or safety concerns from the higher doses.
Page sources
- Higher Doses of CAR-T Therapy Bring Survival Advantage for Young Patients with Hard-to-Treat B-ALL. American Society of Hematology. Retrieved from: https://www.hematology.org/newsroom/press-releases/2022/higher-doses-of-car-t-therapy-bring-survival-advantage. Accessed: 09/21/2022.



