
NOTE: This page was updated 12/19/2022 with information regarding a new clinical study at Abramson Cancer Center testing the idea of a second CAR T-cell infusion for cancer patients.
One of the benefits of CAR T-cell therapy is that many patients only need one infusion of engineered T cells to begin sending cancer into remission. The infused CAR T cells multiply in the body and turn into a “living therapy” that can persist for years.
Scientists are wondering whether some patients may benefit from a second infusion of CAR T cells to help the immune system keep the cancer in retreat.
Memorial Sloan Kettering Cancer Center in New York is hosting a phase 2 clinical trial examining this theory. Penn Medicine’s Abramson Cancer Center is also hosting a clinical trial looking into whether a second infusion of CAR T cells can help patients who don’t benefit from the first attempt.
The study at Memorial Sloan Kettering will administer a second infusion of the CAR T-cell therapy Kymriah® (tisagenlecleucel) for children and young adults with acute lymphoblastic leukemia. Abramson Cancer Center doctors created a new CAR T-cell therapy secreting the interleukin 18 (IL-18) protein to treat non-Hodgkin lymphoma.
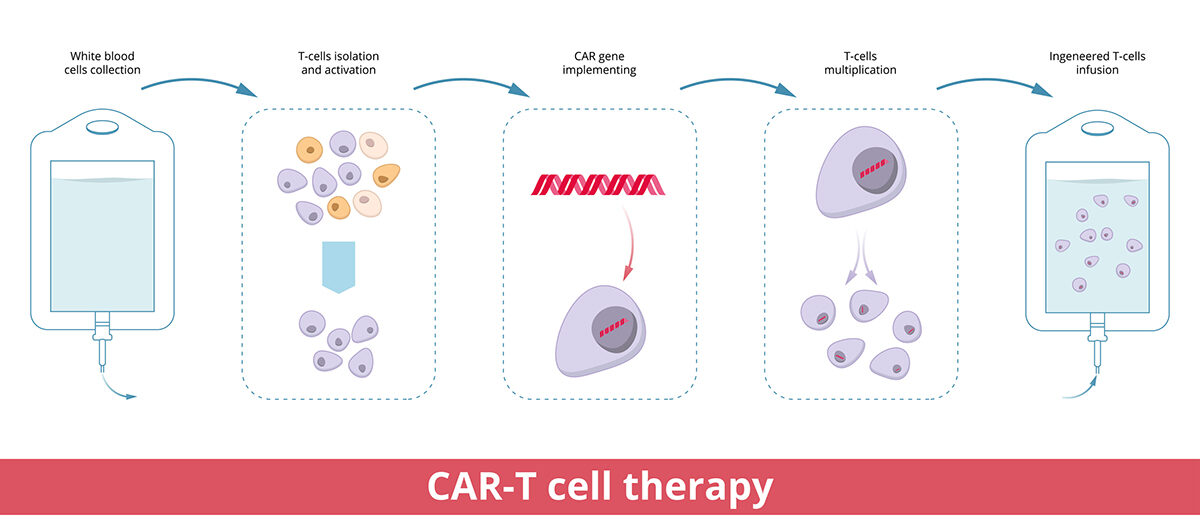
CAR T-cell therapy is a type of cell and gene therapy changing how blood cancers are treated for many patients. Doctors remove T cells from a patient and send them to a laboratory, where scientists create a special protein receptor (called a chimeric antigen receptor). This new protein receptor is added to the T cells to direct their focus to cancer cells expressing the associated protein.
Once the T cells are turned into CAR T cells – and after they’ve multiplied to increase the potency – they’re infused back into the patient’s bloodstream.
Some patients have received CAR T-cell therapy twice — and successfully. Austin Schuetz, who is now 10 years cancer-free from his acute lymphoblastic leukemia, received the second half of his CAR T cells around six months after his first infusion.
History of CAR T-cell therapy for leukemia and lymphoma
The U.S. Food and Drug Administration (FDA) approved Kymriah more than five years ago for acute lymphoblastic leukemia (ALL) in people up to age 25. It was the first CAR T-cell therapy ever approved.
Leukemia is a fast-growing blood cancer in which B cells (blood cells) mutate, replicate, and grow uncontrollably. This leads to having too many B cells in bone marrow and the blood. Kymriah is a CAR T-cell with a protein receptor specifically for a protein found on B cells.
The FDA approval of Kymriah came after years of research and a successful clinical trial that gave widespread credibility to cancer cell and gene therapy. Carl June, MD, (University of Pennsylvania) is credited with developing this CAR T-cell therapy for ALL. The research and development by Dr. June was funded by Alliance for Cancer Gene Therapy, as one of ACGT’s first Research Fellows.
Dr. June is now a member of the ACGT Scientific Advisory Council along with 15 other thought leaders in the field of cell and gene therapy. Your donation to Alliance for Cancer Gene Therapy empowers our organization and the Scientific Advisory Council to fund other research programs that may lead to similar breakthroughs in cancer treatment.
Since the approval of Kymriah, there have been five additional CAR T-cell therapies approved for other blood cancers, such as non-Hodgkin lymphoma (NHL). There are two therapies — Yescarta and Breyanzi — approved as second-line options for patients with NHL cancer. Tecartus, another CAR T-cell therapy, is approved for patients with mantle cell lymphoma.
Details of the new CAR T-cell studies
Doctors at Memorial Sloan Kettering Cancer Center will administer a second infusion of Kymriah 1-2 months after the first infusion. The goal is to achieve an effect of CAR T-cell therapy called “B-cell aplasia” for longer.
B-cell aplasia is when there is a low number of B cells in the bloodstream. Kymriah has a protein receptor directing it to attack all B cells – cancerous and healthy – with a specific protein receptor.
The study is for people ages 25 and younger, imitating the FDA’s approval in 2017. Other patient eligibility criteria includes:
- Enough CAR T cells for a second infusion
- Patients in B-cell aplasia and their acute lymphoblastic leukemia in remission
- Recovered from serious side effects caused by the first infusion
For more information on this clinical trial, visit the Memorial Sloan Kettering Cancer Center website.
Abramson Cancer Center tests new CAR T-cell therapy
The clinical trial at Abramson Cancer Center is a bit different.
Doctors are giving the IL-19 CAR T-cell therapy to non-Hodgkin lymphoma patients who didn’t respond long-term to their first try with CAR T-cell therapy. Either they did not respond to the treatment or relapsed after a short-term response.
Dr. June, who works at Abramson Cancer Center, was the senior author for the preclinical research into the IL-18 therapy.
“We designed an ‘armored’ CAR that secretes IL18 and tested it in mice, where we found it to have potent antitumor efficacy in our preclinical studies,” said Dr. June, who is the Richard W. Vague Professor in Immunotherapy in the Department of Pathology and Laboratory Medicine in the Perelman School of Medicine and Director of the Center for Cellular Immunotherapies at Abramson Cancer Center.
Seven patients received the therapy as of December 2022. The new CAR T-cell therapy — called huCART19-IL18 — worked for all seven (four had a complete response and three had a partial response). All seven were alive at a follow-up of eight months, and none of the patients with a complete response have relapsed with any sign of cancer returning.
For more information about this clinical trial, visit the Abramson Cancer Center website.



