The U.S. Food and Drug Administration (FDA) made history on Feb. 16 by approving a cell therapy for the treatment of advanced melanoma. The treatment is called “tumor-infiltrating lymphocytes therapy” (TIL therapy), and it is the first cell therapy approved to treat a solid tumor.
Alliance for Cancer Gene Therapy (ACGT) compiled a list of questions and answers to help patients and caregivers understand how TIL therapy fights cancer, how scientists make the therapy, and more.
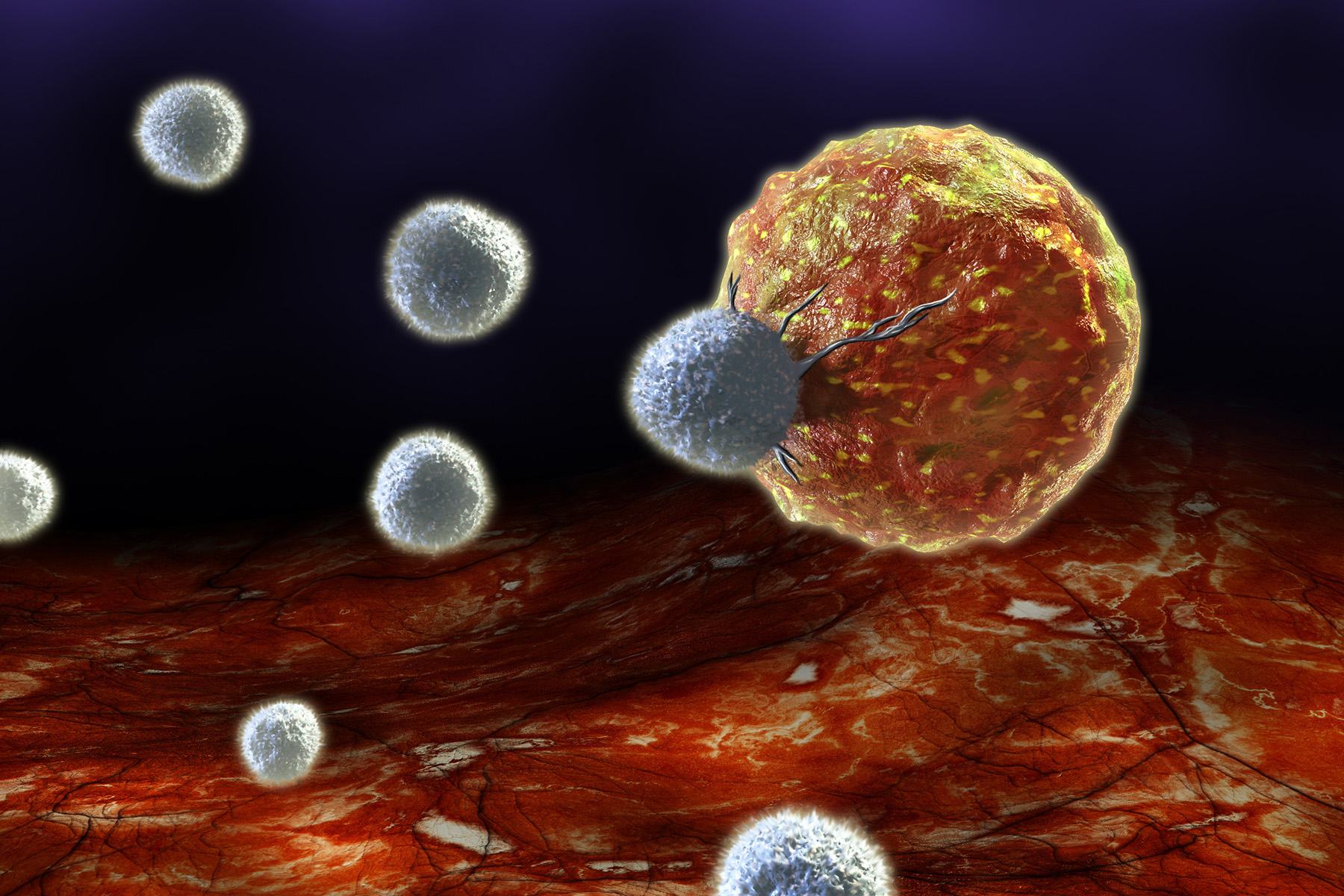
What are tumor-infiltrating lymphocytes?
Tumor-infiltrating lymphocytes, or TILs for short, are white blood cells made in bone marrow that have migrated from the blood to the tumor. People with cancer have TILs, but not enough to destroy their cancer. The cells also become exhausted and ineffective over time.
What is TIL therapy?
TIL therapy is a cancer cell therapy that involves multiplying the lymphocytes to provide the patient with more cells capable of attacking their tumor. Read more about TIL therapy on The University of Texas MD Anderson Cancer Center’s website.
What is the process for making TIL therapy?
TIL therapies are created by collecting a sample of the patient’s tumor, removing the TILs in the tumor sample, multiplying them, and returning the cells to the patient. Learn more about the process on the Moffitt Cancer Center’s website.
Are there any side effects from TIL therapy?
According to the National Cancer Institute, TIL therapy does not cause any severe side effects. Potential manageable side effects include fever, chills and shortness of breath for up to two weeks following the infusion of TILs.
What is TIL therapy approved for?
The FDA recently (2024) approved a TIL therapy called lifileucel for the treatment of unresectable or metastatic melanoma, a skin cancer diagnosed in approximately 100,000 people in the U.S. each year. Eligibility for lifileucel requires that patients previously received a PD-1 blocking antibody and, if BRAF V600-positive, a BRAF inhibitor with or without a MEK inhibitor.
Read more about the approval on the FDA’s website.
Who manufactures lifileucel?
Lifileucel is manufactured by Iovance Biotherapeutics, Inc., and sold under the brand name Amtagvi.
Why did the FDA approve Amtagvi for advanced melanoma?
The FDA’s approval of Amtagvi is based on the results of a phase 2 clinical trial. Among 73 people in the study, 23 (31.5%) had at least a partial anti-tumor response and three patients had complete responses (meaning there was no sign of cancer on scans).

How will this FDA approval help people with advanced melanoma?
People with metastatic melanoma usually have already tried other treatments, such as chemotherapy and immunotherapy, with no long-term success. According to the Melanoma Research Alliance, the 5-year survival rate of metastatic melanoma (stage 4) is approximately 22%.
This approval will give patients another treatment option to control and hopefully defeat their cancer.
How can people with melanoma receive Amtagvi?
People diagnosed with melanoma should speak with their oncologist about treatment options and whether they are eligible for Amtagvi. ACGT has tips for how to talk with a doctor about cancer treatment.
Can TIL therapy be used for other types of cancer?
Scientists at the top cancer centers are researching how to develop TIL therapies for other solid tumors. ACGT is currently funding research to develop a TIL therapy for pancreatic cancer, which is diagnosed in approximately 66,000 people in the U.S. each year and has a 5-year survival rate of only 13%.
Please sign up for a free monthly email newsletter and other communications from ACGT to learn more about TIL therapy along with other cancer cell and gene therapies. ACGT’s mission is to fund innovative scientists and biotechnology companies working to harness the power of cell and gene therapy to transform how cancer is treated and to drive momentum toward a cure.
Page sources
- TIL therapy: 6 things to know. National Cancer Institute. Retrieved from: https://www.cancer.gov/news-events/cancer-currents-blog/2024/fda-amtagvi-til-therapy-melanoma. Accessed: 03/15/2024.
- Breakthrough Treatment for Solid Tumors With TIL Therapy. University of Maryland Medical Center. Retrieved from: https://www.umms.org/ummc/pros/physician-briefs/cancer/immunotherapy/til-therapy. Accessed: 03/15/2024.



