
Cancer cell and gene therapy is an emerging form of treatment already approved for some blood cancers and is in testing for solid tumors.
However, not many people know what cell and gene therapy actually is, how it works to kill cancer cells, some of the different types of cell and gene therapy, and other important aspects.
Alliance for Cancer Gene Therapy (ACGT) has compiled some of the most common questions patients have about cell and gene therapy, along with useful answers to empower you to better understand this field of cancer treatment.
What is cancer cell and gene therapy?
Cancer cell and gene therapy uses the power of a patient’s own immune system to find and destroy cancer cells without hurting healthy tissue. Scientists modify the DNA within a patient’s existing immune cells, such as T cells, to give those cells new instructions on how to find and fight cancer.
What is cell therapy for cancer?
Cell therapy for cancer involves modifying cells to improve the immune system’s ability to fight cancer. According to the Dana Farber Cancer Institute, scientists collect a specific set of cells from the blood, modify them to produce a more vigorous attack on a patient’s cancer cells, and then reinject them into the patient.
What is cancer gene therapy?
The National Cancer Institute defines gene therapy as an experimental treatment that adds a new gene to the body’s cells or replaces or repairs a mutated (changed) gene inside cells to help prevent or treat cancer.
What are the different types of cancer gene therapy?
There are four main types of cancer gene therapy: gene editing; gene replacement; gene addition; and gene inhibition. Gene editing corrects the cancer cell’s gene to remove any mutation. Gene replacement replaces a faulty gene in a cancer cell with a healthy version to fix the cell. Gene addition inserts a new piece of genetic code, usually to an immune cell, to help it destroy cancer. Gene inhibition, or deletion, simply shuts down the faulty gene within a cancer cell.
What are some examples of cell and gene therapy?
There are a few different types of cell and gene therapy. They include:
- CAR T-cell therapy
- Engineered T-cell receptors
- Tumor-infiltrating lymphocytes
- Oncolytic viruses
- Dendritic cell therapy
Which cell and gene therapies are approved by the FDA?
There are seven cell and gene therapies approved by the U.S. Food and Drug Administration (FDA) to treat various cancers. There are six CAR T-cell therapies approved to treat blood cancers such as leukemia, lymphoma and multiple myeloma.
- Kymriah (acute lymphoblastic leukemia, large B-cell lymphoma, and follicular lymphoma)
- Breyanzi (non-Hodgkin lymphoma)
- Yescarta (non-Hodgkin lymphoma)
- Tecartus (mantle cell lymphoma and acute lymphoblastic leukemia)
- Abecma (multiple myeloma)
- Carvykti (multiple myeloma)
There is one engineered T-cell receptor therapy (Kimmtrak) approved to treat a rare skin cancer of the eye, called uveal melanoma.
Visit our approved cell and gene therapies page for more details about each approval and eligibility requirements for patients to receive these therapies.
What is CAR T-cell therapy?
CAR T-cell therapy is a type of cell and gene therapy that genetically alters immune cells called T cells to destroy cancer cells more effectively. CAR stands for “chimeric antigen receptor.” Doctors can remove patients’ T cells to have a chimeric antigen receptor added. Scientists develop the receptors to link with proteins highly expressed on cells of a specific cancer.
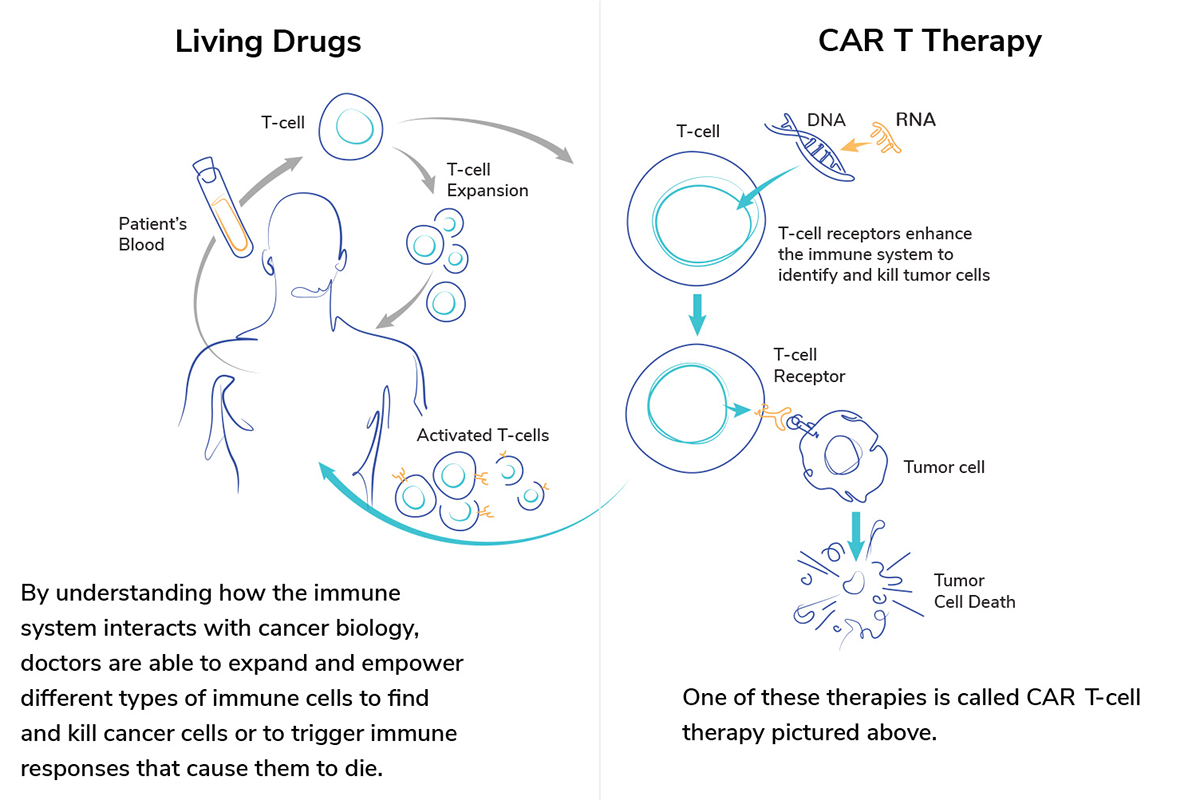
Where can I go to receive CAR T-cell therapy?
Many of the top cancer centers and hospitals offer CAR T-cell therapy treatment. For patients interested in CAR T-cell therapy to treat their cancer, please visit this resource for a directory of all medical institutions offering at least one of the FDA-approved CAR T-cell therapies to patients.
What are engineered T-cell receptors?
Engineered T-cell receptors are protein receptors added to our T cells to help them find and destroy cancer cells. Engineered T-cell receptors are similar to CAR T cells. The major difference is engineered T-cell receptors look for fragments of cancer proteins rather than a whole protein.
What are tumor-infiltrating lymphocytes?
Tumor-infiltrating lymphocytes are white blood cells that have shown they are capable of fighting cancer. These cells have migrated from the patient’s blood to the tumor and have begun infiltrating through the outer wall of the cancer cell. Tumor-infiltrating lymphocytes can be removed from the patient’s body and multiplied to create more of these effective cancer-fighting cells.
What are oncolytic viruses?
Oncolytic viruses are engineered forms of viruses used to infect and kill cancer cells. The use of oncolytic viruses is called “virotherapy” or “oncolytic virus therapy.” The engineered viruses are taught to only infect cancer cells and leave healthy tissue alone, which makes them safe. Once the oncolytic viruses are inside of cancer cells, they multiply and spread to other nearby cancer cells. The goal is to break the cells apart from within.
What are dendritic cells?
The National Cancer Institute defines dendritic cells as a special type of immune cell found in tissues, such as the skin, and boosts immune responses by showing antigens on its surface to other cells of the immune system. They can help the immune system learn how to identify cancer cells. Doctors can modify the body’s dendritic cells to improve the immune response to cancer. This is called dendritic cell therapy.
Are there any cancer cell and gene therapies in clinical trials?
Yes. There are clinical trials featuring cell and gene therapies for cancer. As of June 2022, according to an article on Fierce Pharma, there are more than 1,000 cell and gene therapy clinical trials underway globally, with the vast majority of studies in phase 1 or phase 2.
Visit the ACGT clinical trials page for help finding a cell and gene therapy trial. We also recommend visiting the clinicaltrials.gov website for a list of all clinical trials.



